Rosiglitazone_Enantiomers_Structural_Formulae Tags: Rosiglitazone View |
Rosiglitazone is an anti-diabetic drug in the thiazolidinedione class of drugs. It works as an insulin sensitizer, by binding to the PPAR receptors in fat cells and making the cells more responsive to insulin. It is marketed by the pharmaceutical company GlaxoSmithKline as a stand-alone drug (Avandia) and in combination with metformin (Avandamet) or with glimepiride (Avandaryl). Annual sales peaked at approx $2.5bn in 2006, but declined after reports of adverse effects. The drugs patent expires in 2012.
Some reports have suggested that rosiglitazone is associated with a statistically significant risk of heart attacks, but other reports have disagreed, and the controversy has not been resolved. Concern about adverse effects has reduced the use of rosiglitazone despite its sustained effects on glycemic control.
The drug was controversial in the U.S. Food and Drug Administration. Some reviewers concluded that rosiglitazone caused more deaths than pioglitazone (Actos) and recommended that rosiglitazone be taken off the market, but an F.D.A. panel disagreed and it remains on the market in the U.S.
Verifiedrevid: 307919932
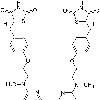
Iupac Name: (''RS'')-5-4-(2-methyl(pyridin-2-yl)aminoethoxy)benzylthiazolidine-2,4-dione
Imagename: 1 : 1 mixture (racemate),
Casno Ref: cascite
Casno Ref: cascite
Number on List: 122320-73-4
Chemspiderid: 70383
Atc Prefix: A10
Atc Suffix: BG02
Pubchem: 77999
Iuphar Ligand: 1056
Drugbank: APRD00403
C: 18 H = 19 N = 3 O = 3 S = 1
Molecular Weight: 357.428 g/mol
Smiles: OC(=O)C(Cc1ccc(OCCc2nc(oc2C)c2ccccc2)cc1)Nc1ccccc1C(=O)c1ccccc1
Bioavailability: 99%
Protein Bound: 99.8%
Metabolism: Hepatic (Liver) (CYP2C8-mediated)
Elimination Half-life: 3-4 hours
Excretion: Renal (Kidney) (64%) and fecal (23%)
Licence Eu: Avandia
Licence Us: Rosiglitazone
Pregnancy Au: B3
Pregnancy Us: C
Legal Uk: POM
Legal Us: Rx-only
Routes Of Administration: Oral