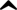
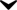
Modafinil (Provigil, Alertec, Modavigil, Modalert, Modiodal, Modafinilo, Carim) is an analeptic drug manufactured by Cephalon, and is approved by the U.S. Food and Drug Administration (FDA) for the treatment of narcolepsy, shift work sleep disorder, and excessive daytime sleepiness associated with obstructive sleep apnea.
In South America the drug compound is called Modafinilo and is marketed in Uruguay, Ecuador and Columbia under the brand name Carim by Roemmers Pharmaceuticals in Uruguay,
Despite extensive research into the interaction of modafinil with a large number of neurotransmitter systems, a precise mechanism or set of mechanisms of action remains unclear. It seems that modafinil, like other stimulants, increases the release of monoamines, specifically the catecholamines norepinephrine and dopamine, from the synaptic terminals. However, modafinil also elevates hypothalamic histamine levels, leading some researchers to consider Modafinil a "wakefulness promoting agent" rather than a classic amphetamine-like stimulant (as evidenced by the difference in c-Fos distribution caused by modafinil as compared to amphetamine). Despite modafinils histaminergic action, it still partially shares the actions of amphetamine-class stimulants due to its effects on norepinephrine and dopamine.
An NIAAA study highlighted "the need for heightened awareness for potential abuse of and dependence on modafinil in vulnerable populations" due to the drugs effect on dopamine in the brains reward center. However, the synergistic actions of modafinil on both catecholaminergic and histaminergic pathways lowers abuse potential as compared to traditional stimulant drugs while maintaining the effectiveness of the drug as a wakefulness promoting agent. Studies have suggested that modafinil "has limited potential for large-scale abuse"cite journaltitle=Modafinil: Preclinical, Clinical, and Post-Marketing Surveillance?A Review of Abuse Liability Issues journal=Annals of Clinical Psychiatrydate=2004-04-01first=Hughlast=Myrickvolume=16issue=2pages=101-9doi= 10.1080/10401230490453743url= and "does not possess an addictive potential in naive new individuals."cite journaltitle=Study of the addictive potential of modafinil in naive and cocaine-experienced ratsjournal=Psychopharmacologiayear=2002first=DEROCHE-GAMONETlast=V.volume=161issue=4pages=387-395id= url=Lelast6=P.first6=Piazza
Although there was some speculation modafinil could be effective in the treatment of attention-deficit hyperactivity disorder (ADHD) due to its similarity to amphetamine-class stimulants, in 2006 it was specifically found by the FDA to be unfit for use by children for that purpose. Cephalons own label for Provigil now discourages its use by children for any purpose. Other potentially effective, but unapproved targets include the treatment of depression (major depressive disorder), cocaine dependence, Parkinsons disease, schizophrenia, and disease-related fatigue (Fatigue (medical)).
Under the US Food and Drug Act, drug companies are not allowed to market their drugs for off-label uses (conditions other than those officially approved by the FDA); Cephalon was reprimanded in 2002 by the FDA because its promotional materials were found to be "false, lacking in fair balance, or otherwise misleading". Cephalon plead guilty to a criminal violation and paid several fines, including $50 and $425 million fines to the U.S. government in 2008 due to its marketing.
In addition to being used for narcolepsy, sleep-apnea, and shift-work sleep disorder, modafinil has been considered for use in the treatment of parkinsons disease, fatigue symptom treatment, multiple sclerosis, and cocaine addiction. Modafinil has received attention regarding its wide range of potential clinical applications.
Modafinil and its chemical precursor adrafinil were developed by Lafon Laboratories, a French company acquired by Cephalon in 2001. Modafinil is the primary metabolite of adrafinil, and, while its activity is similar, adrafinil requires a higher dose to achieve equipotent effects. Modafinil is a racemic mixture; the (R)-enantiomer is known as armodafinil (Nuvigil).
Watchedfields: changed
Verifiedrevid: 306562054
Iupac Name: (�)-2-di(phenyl)methylsulfinylacetamide
Image2: Modafinil3d.png
Casno Ref: cascite
Number on List: 68693-11-8
Atc Prefix: N06
Atc Suffix: BA07
Pubchem: 4236
Drugbank: DB00745
Chemspiderid: 4088
C: 15 H = 15 N = 1 O = 2 S = 1
Molecular Weight: 273.35 g/mol
Smiles: NC(=O)CS(=O)C(C1=CC=CC=C1)C1=CC=CC=C1
Bioavailability: Not determined due to the aqueous insolubility
Protein Bound: 60%
Metabolism: Hepatic, including CYP3A4 and other pathways
Elimination Half-life: 12-15 hours
Excretion: Urine (as metabolites)
Pregnancy Au: B3
Pregnancy Us: C
Legal Au: S4
Legal Ca: Rx-only
Legal Uk: POM
Legal Us: Schedule IV
Routes Of Administration: Oral


