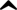
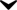
Alendronic acid (INN (International Nonproprietary Name)) or alendronate sodium (USAN (United States Adopted Name), sold as Fosamax by Merck (Merck & Co.)) is a bisphosphonate drug (medication) used for osteoporosis and several other bone diseases. It is marketed alone as well as in combination with vitamin D (2,800 U and 5600 U, under the name Fosamax+D). Mercks U.S. (United States) patent on alendronate expired in 2008 and Merck lost a series of appeals to block a generic version of the drug from being certified by the U.S. Food and Drug Administration (Food and Drug Administration (United States)) (FDA).
On February 6, 2008, the US FDA approved the first generic versions of alendronate, which were marketed by Barr Pharmaceuticals and Teva Pharmaceuticals USA. Teva Pharmaceuticals manufactures generic alendronate in 5-milligram, 10-milligram, and 40-milligram daily doses, and in 35-milligram and 70-milligram weekly doses, while Barr made generic alendronate in 70-milligram tablets, which were taken once weekly. Barr pharmaceuticals were subsequently acquired by Teva in July 2008.
Iupac Name: sodium 4-amino-1-hydroxy-1-(hydroxy-oxido-phosphoryl)- butylphosphonic acid trihydrate
Image2: Alendronate_sf.png
Inchi: 1/C4H13NO7P2/c5-3-1-2-4(6,13(7,8)9)14(10,11)12/h6H,1-3,5H2,(H2,7,8,9)(H2,10,11,12)
Inchikey: OGSPWJRAVKPPFI-UHFFFAOYAU
Number on List: 121268-17-5
Chemspiderid: 2004
Atc Prefix: M05
Atc Suffix: BA04
Pubchem: 2088
Drugbank: APRD00561
C: 4 H=18 N=1 Na=1 O=10 P=2
Molecular Weight: 325.124
Smiles: O=P(O)(O)C(O)(CCCN)P(=O)(O)O
Bioavailability: 0.6%
Metabolism: excreted unchanged
Elimination Half-life: 126 months
Excretion: renal
Pregnancy Category: C
Legal Status: POM (UK), Rx-only (US)
Routes Of Administration: Oral


