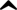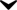alcohol is any organic compound in which a hydroxyl group (Functional group) (-O (oxygen)H (hydrogen)) is bound to a carbon atom of an alkyl or substituted alkyl group. An important group of alcohols is formed by the simple acyclic (Aliphatic compound) alcohols, the general formula for which is CnH2n+1OH. Of those, ethanol (C2H5OH) is the type of alcohol found in alcoholic beverages, and in common speech the word alcohol means, specifically, ethanol.
Other alcohols are usually described with a clarifying adjective, as in isopropyl alcohol (propan-2-ol) or wood alcohol (methyl alcohol, or methanol). The suffix -ol appears in the IUPAC chemical name (IUPAC nomenclature of organic chemistry) of all alcohols.
There are three major subsets of alcohols: primary (1�), secondary (2�) and tertiary (3�), based upon the number of carbon atoms the C-OH groups carbon (shown in red) is bonded to. Ethanol is a simple primary alcohol. The simplest secondary alcohol is isopropyl alcohol (propan-2-ol), and a simple tertiary alcohol is tert-butyl alcohol (tert-Butanol) (2-methylpropan-2-ol).